The shapes of atomic orbitals
If we work on a spherical base which is the most likely to be compared to an atom, as a sphere represents the maximum of volume distribution, this is why bubbles aren't square or pyramidal, and depending on the spherical base variables r,θ and φ, the equation is often written this way: 𝛙(r,θ,φ)= R(r)*Y(θ,φ).
To solve this equation we need some set of data like mass and that describes a type of atom like hydrogen. By applying the necessary calculus we find that the resolution of this equation requires the insertion of 3 numbers within the equation which is known as the Quantum Numbers (n,l,m) .also there seem to be some mathematical restrictions for these numbers to have a physical sense, which are : n > 0
0 ≤ l ≤ n-1
-l ≤ m ≤ l
Let's start by clarifying the significance of each of these parameters:
n: The main quantum number: it is a positive integer intervening in the resolution of R(r), It defines the energy of the electron like the case of hydrogen En = -13,6. Z² / n² (ev).
Also, the size of the orbital (its volume) depends on this number
n: 1 2 3 4
layer: K L M N
l: Secondary quantum number: it fixes the shape of the orbital and takes all the integer volumes from 0 to n-1 so one can say that at a level n corresponds n under levels or under layers or states named according to l
l= 0 1 2 3s p d f
Let's consider this example quickly, you have to just follow the rules:
n=1; l=0 ; state 1s
n=2; l=0; state 2s
; l=1; state 2p
n=3; l=0 ; state 3s
; l=1; state 3p
;l=2; state 3d
And so on...
m: Magnetic quantum number: intervenes in the resolution of the angular function. It sets the orientation of the atomic orbital with respect to a reference direction and must verify
-l ≤ m ≤ l
If for example l=0; m=0
but if l=1 the m can be 1,-1 or 0 where each one is attributed to a single direction (ox) (oy) and (oz)
I am not going to any further equations, you can find this somewhere else on the internet.
The real question is what does the resolution of the equation 𝛙(r,θ,φ)= R(r)*Y(θ,φ) give us?
In fact, As the variables suggest, it's a sort of a three-dimensional graph, it's a specific volume of space surrounding the nucleus where every layer (s,p,d,f) has its own unique shape in various directions.
This size shows all the possible orbitals in all directions based on the magnetic quantum number m.
You can see that there are a lot of different shapes so I am going to rely on some examples of simple atoms. Nothing is easier than studying the hydrogen atom (Z=1), knowing that every shape can only support a pair of electrons, the electron goes to the s orbital so it's 1s¹. For Brome (Z=5) two electrons occupy each one of the s orbitals that belongs to the two energy levels while the fifth one occupies one direction of the p orbital (ox),(oy) or (oz) so it's 1s²2s²2p⁶.
For Neon (Z=10) the p orbital contains 6 électrons in all the three dimensions, 2 for each one
so the final structure is 1s²2s²2p⁶.
For Sodium (Z=11) the extra electron goes to the s orbital of the 3rd layer and it becomes 1s²2s²2p⁶3s¹, the 3s sphere encompasses all the other shapes and so on the more, the number of electrons increases the more complex the 3d orbitals become.
But what do these shapes really signify?
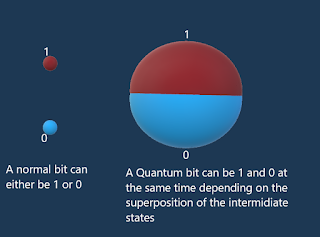
The answer is: they represent the volume of space where there is a high probability of finding the electrons, it's the non-superposed images of the electrons before the active measurement, it's analogue to the pattern of interference on a screen.
Now all things make sense !!





Comments
Post a Comment