The awakening of the Quantum Theory !
The classic models of the atom (the Rutherford-Bohr model) was incapable of explaining the details of the emission spectrum of poly electronic atoms until another revolutionary theory emerged making even the elite of physicists like Albert Einstein live in a constant nightmare, the theory was nothing else than the famous Quantum mechanics which renewed our whole vision to the world and especially the understanding of the motion of subatomic particles.
This began when physicist Louis de Broglie postulated that every corpuscle of matter even the newly discovered electrons are associated with a wave that accompanies its movement. if the electron with a mass m and a velocity V has a momentum p=m.V, the associated wave will be 𝛌= h/p = h/m.V.
In Bohr's model, the electron is a particle that moves in a circular uniform trajectory around the nucleus, we call this kind of trajectory a stationary orbital to which we attribute a number n depending on its order from the inside of the atom to the outside. However, in Broglie's model, the electron describes a wave like motion instead of a perfect circular motion with a wavelength 𝛌 where 2𝚷r =n.𝛌 = n.h / m. V. knowing that 2𝚷r is the perimeter of the circle, the equation means that the total length of the orbit is equal to an integer of 𝛌.
This equation works everywhere but we don't quite realise the effect because the variations are so small that we cannot perceive them.
To clarify the idea let's proceed to some mathematical examples:
If a macroscopic particle, let's say a tennis ball has a mass m=50g and a velocity V= 40 m/s and knowing that h which is the Planck constant is equal to 6,62.10 exp(-34)
By applying the Broglie's equation 𝛌 = 6,62.10 exp(-34) / 50 . 10 esp(-3) . 40 = 3,32. 10 exp(-34)
This result has not a physical sense. In fact, at the microscopic scale, the unit of measure is of the order of Angstroms (Ǻ). I mean how can we perceive such a tiny variance in a Wave? It's tending to zero so even if it exist's in everyday's objects it remains unnoticed. So what's the point?
Don't worry here's the magic, Let's consider another example but this time the studied particle is a microscopic electron where its mass m=9,108. 10 exp(-31) Kg and velocity V= 10 exp(7) m/s.
According to the same calculations 𝛌= 0,736 Ǻ.
Here everything changes, the wavelength is noticeable and very comparable to the atomic magnitudes, it's no longer neglected, it has a strictly physical sense because everything is relative.
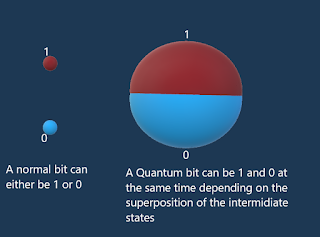
This is what revolutionized the classical physics, the wave like motion of the electrons is so relatively important that it cannot be disregarded and here we are forced to admit that electrons have 2 undeniable aspects: a corpuscle and a wave.


Comments
Post a Comment