Heat and Temperature
In the previous article, we learned that temperature helps somehow to cool the motion of qubits making it easier to manipulate them and avoiding the risk of quantum decoherence so how does it actually work?
The answer is in the question itself, all we need to do is understand the notions of heat and cold and it's very easy. Well, cold is simply the absence of heat however heat is a type of energy that causes particles to agitate unbalancing the system.
This kind of energy is special, it's what makes you feel the coldness of a refrigerator or the warmness of a mug of coffee and it's directly linked to temperature.
Temperature is the measure of how much kinetic energy in a system, it's not really a measuring because you can't say "this object is 298°K", it's more like a comparison to a beforehand fixed value which is the temperature of water freezing to which we attributed the value 0°C., On the other hand, we attributed the value of 100°C to the temperature of water vaporization. Each other measured temperature is compared to this scale divided by 100 to obtain a single degree Celsius. Of course, there are other units of measurement we just need to apply the converting formula.
This isn't the main problem, however now we know that heat is related to temperature and moreover to kinetic energy.
Let's take the example of water at 0°C or below, the visible state is solid in a shape of an ice cube and this is due to the lack of heat within the system, the particles are quiet and close to each other but what happens if the temperature rises? In this case, the state of the system transforms bit by bit to a liquid state characterized by more fluidity where particles are more agitated and far from each other.
By raising the temperature above 100°C the state sudden another change from liquid to a gas, the particles are now more and more separated from each other trying to occupy space as much as possible according to diffusion laws from the lower gradient to the higher one.
The increasing of particles motion within the heated object is usually paired with a rise in volume or length depending on the shape of that specific object so the more heat we bring to a system the more volume it tends to colonize.
Water molecules make somehow the exception in the solidification phase where a cube of ice occupies more volume that it's analog cup of water. But for the rest of the systems, the volume and temperature are proportional.
Have you ever wondered why do bridges possess some metal plates implanted in the concrete or why do train rails have perpendicular joints between them?
These components are called expansion joints and they are installed to prevent the shrinking or dilatation of a structure caused by the temperature's rising, they also add more resistance to the stress and strain caused by the moving vehicles above them.
These types of joints allow the structure to resist these changes by giving them space to gain or lose volume resisting the stress and avoiding fatal fissures. The change of volume is usually expressed by the equation ΔV = β V₀ ΔT where β is the coefficient of volume expansion that varies from a material to another and V₀ and ΔT are respectively the initial volume and the change in temperature.
Temperature doesn't only affect solids, it affects gases as well this can be visualized by applying pressure to air within a syringe while closing the end and maintaining the temperature constant.
The air seems to be easy to compress at first but the more you push the harder it gets because the molecules are more and more close to each other. The Boyle's law translates this proportionality as stated: V ∝ 1/P.
The second experiment consists of taking a balloon filled with air and by heating that air, while maintaining the pressure constant, the temperature rises and as the definition states the kinetic energy of the air molecules rises dramatically making them in a hysterical motion. These molecules collide with each other and also with the inner wall of the container making it stretch allowing the volume of the air to expand according to the Charle's law: V ∝ T
But in another scenario when we put that heated air in another static container like an iron ball, the motion of the air molecules is not even close to stretch the wall but if we measure the inner pressure we can notice that it rises proportionally to the temperature. This is also manifested in the way some casseroles have locks on them because braising food requires a significant amount of time increasing the motion of molecules and, therefore, the pressure within the casserole, the lock is meant to contain that pressure, otherwise, the recipient could explode. The Gay Lussac's law translates this proportionality : P ∝ T.
Based on these 3 proportionalities we can establish the famous resuming law known as the ideal gas law PV = n R T ( R:universal gas constant, n: moles) This formula allows us to study the parameters of an ideal gas like knowing the mole's number within the balloon...
This only applies when a gas is considered Ideal meaning that we only take into consideration the random motion of the particles and their elastic shocks neglecting all other types of interactions.




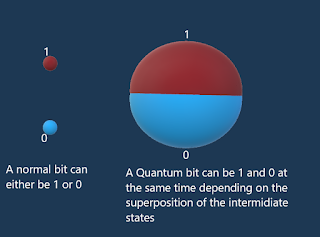
Comments
Post a Comment